Chapter 7 Practice Test
1. Which of the following molecules have the same molecular geometry
as SbCl3? (Circle all)
a. AlH3 b. NH3 c. H2CO d. PI3
2. The molecule KF2 has ________ electron geometry, __________ molecular geometry, and ___________ orbital hybridization.
3. Draw the Lewis structure and at least one resonance structure for
SCN-
4. Draw the Lewis structure of XeF4 and determine;
a. The orbital hybridization is ____________
b. The molecular geometry is _____________
c. The electron geometry is _______________
5. In each pair, circle the most polar bond.
a. N-H or N-F b. C-Cl or O-Cl c. Se-F or S-F
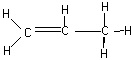
6. The drawing shown below has _____ sigma bonds and ______ pi bonds.