Some important links
NIST Chemistry WebBook
Smokeless Powder Database
Interfire Arson Database
Ignitable liquids Database
Mitochondrial Database
NIST Short Tandem Repeats (STR) Database
Y-Haplotype Reference Database (YHRD)
American Academy of Forensic Science (AAFS)
Society of Forensic Toxicologists (SOFT)
National Institute of justice (NIJ)
Drug Enforcement Administration (DEA)
Information on some Analytical Techniques
Capillary Electrophoresis
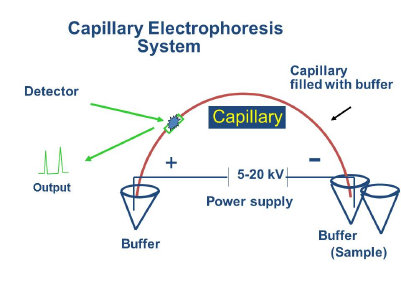 It is an analytical technique in which separation occurs in silica capillaries based on size to charge ratio of ions under the influence of an applied electric field. Unlike other separation techniques which involve partitioning between mobile and stationary phases, CE separtions are in a single liquid phase and produce much less diffusive bandbroadening. Another advantage of the technique is the development of electrically induced osmotic flow that results from wall charges in the silica tubes. These electrically induced flows permit the movement of bulk solutions without the necessity of mechanical pumping, making CE sytems ideal for microscale devices. Sample volumes and solvents needs are reduced and making the procedure inexpensive and practical for detection of trace quantities of materials. Typical detection modes include UV/Vis, fluorescence, amperometric and mass spectrometry.
It is an analytical technique in which separation occurs in silica capillaries based on size to charge ratio of ions under the influence of an applied electric field. Unlike other separation techniques which involve partitioning between mobile and stationary phases, CE separtions are in a single liquid phase and produce much less diffusive bandbroadening. Another advantage of the technique is the development of electrically induced osmotic flow that results from wall charges in the silica tubes. These electrically induced flows permit the movement of bulk solutions without the necessity of mechanical pumping, making CE sytems ideal for microscale devices. Sample volumes and solvents needs are reduced and making the procedure inexpensive and practical for detection of trace quantities of materials. Typical detection modes include UV/Vis, fluorescence, amperometric and mass spectrometry.
One great advantage of CE analysis is its flexibility A single instrumental setup can be used to separate everything from small ions to large proteins. Changing the composition of the buffer permits this wide range of separation. By adding detergents to the buffer, one can separate neutral molecules due to their hydrophobic interaction with micelles created in the buffer. Addition of crown ethers or cyclodextrins produces guest/host interactions that permit even more selective separations. Highly efficient separation of of enantiomers is quick and easy with this technique. Addition of water soluble polymers permits size based separation of DNA and proteins, and affinity interactions can also be used for selective isolation or kinetic measurements.
Real Time PCR
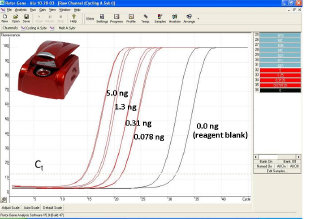 Real time PCR is the most efficient way forensic laboratories quantify the amount of DNA extracted from biofluids left behind at crime scenes. Real time PCR has nearly 5 orders of magnitude dynamic range and detection is easily automated. The system operates by detection of changes in fluorescence as the amount of DNA increases. By measuring the relative rate of increase of the number of DNA copies at an early stage of the process, the initial quantity of sample can be estimated based on the point at which the fluorescence crosses a thershold point known as Ct or cycle threshold. The real time approach is far better than more traditional endpoint detection procedures as the reagents are fresh and quantities are relatively low, avoiding problems with limiting reagents and well to well temperature variations. There are three major techniques used in real time PCR, fluorescence intercalation, Taqman, and Plexor. Intercalation is the most sensitive procedure as it labels DNA in multiple locations and can also be used with loci such Alu inserts which exists in multple copies across the genome. Alternatively the Taqman and Plexor procedures permit multiple targets to be analyzed permitting the simultaneous determination of autosomal, Y and internal standard DNA.
Real time PCR is the most efficient way forensic laboratories quantify the amount of DNA extracted from biofluids left behind at crime scenes. Real time PCR has nearly 5 orders of magnitude dynamic range and detection is easily automated. The system operates by detection of changes in fluorescence as the amount of DNA increases. By measuring the relative rate of increase of the number of DNA copies at an early stage of the process, the initial quantity of sample can be estimated based on the point at which the fluorescence crosses a thershold point known as Ct or cycle threshold. The real time approach is far better than more traditional endpoint detection procedures as the reagents are fresh and quantities are relatively low, avoiding problems with limiting reagents and well to well temperature variations. There are three major techniques used in real time PCR, fluorescence intercalation, Taqman, and Plexor. Intercalation is the most sensitive procedure as it labels DNA in multiple locations and can also be used with loci such Alu inserts which exists in multple copies across the genome. Alternatively the Taqman and Plexor procedures permit multiple targets to be analyzed permitting the simultaneous determination of autosomal, Y and internal standard DNA.
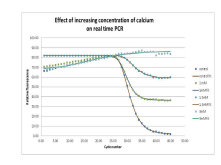 There is more that real time PCR can do besides quantification. We have been interested in the use of real time PCR to predict sample quality. As a result we have been using the technique to develop an understanding of PCR inhibition. By examining the rate of amplification, DNA melt curve effects and the subsequent amplification of STR alleles we can begin to determine the effect of DNA sequence and PCR inhibitors such as humic acid (soils) hematin (blood) bile salts (faeces) and collagen (bone) on the ability of the forensic analysis to amplify and produce a genotype from various crime scene samples.
There is more that real time PCR can do besides quantification. We have been interested in the use of real time PCR to predict sample quality. As a result we have been using the technique to develop an understanding of PCR inhibition. By examining the rate of amplification, DNA melt curve effects and the subsequent amplification of STR alleles we can begin to determine the effect of DNA sequence and PCR inhibitors such as humic acid (soils) hematin (blood) bile salts (faeces) and collagen (bone) on the ability of the forensic analysis to amplify and produce a genotype from various crime scene samples.
Electrospray Mass Spectrometry
 In forensic analysis it is critical to be able to identify a compound sufficiently that the analyst can defend their findings in court. Typically this means combining a separation technique like liquid chromatography with a spectroscopic technique. Electrospray mass spectrometry is compatible with liquid based separations and has a wide range of applications in forensic analysis. The technique involves spraying the sample and eluent through a charged needle. This process produces a cloud of charged droplets (Taylor cone) that gradually evaporate producing smaller even more highly charged droplets. These break up due to coulombic forces and continue evaporating until protonated molecular ions and molecular ion adducts are formed. These adduct ions include sodium, ammonium and in the case of negative ion ESI may include chloride, formate and nitrate ions. Analyte signal is optimized through careful control of eluent parameters including solvent concentration, buffer ion additives and pH.
In forensic analysis it is critical to be able to identify a compound sufficiently that the analyst can defend their findings in court. Typically this means combining a separation technique like liquid chromatography with a spectroscopic technique. Electrospray mass spectrometry is compatible with liquid based separations and has a wide range of applications in forensic analysis. The technique involves spraying the sample and eluent through a charged needle. This process produces a cloud of charged droplets (Taylor cone) that gradually evaporate producing smaller even more highly charged droplets. These break up due to coulombic forces and continue evaporating until protonated molecular ions and molecular ion adducts are formed. These adduct ions include sodium, ammonium and in the case of negative ion ESI may include chloride, formate and nitrate ions. Analyte signal is optimized through careful control of eluent parameters including solvent concentration, buffer ion additives and pH.
Surface Enhanced Raman Spectroscopy
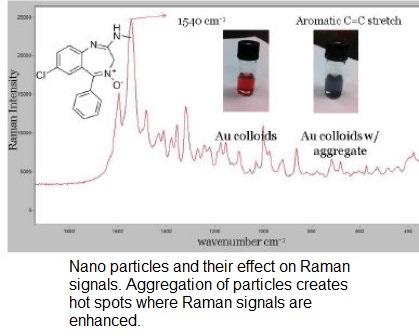 Raman spectroscopy is a spectroscopic technique that produces results based on inelastic scattering of light interacting with molecules of interest. From an analytical standpoint the problem with Raman spectroscopy is its relatively poor sensitivity. However the sensitivity of the procedure can be enhanced through interactions with roughened metal surfaces or nanoparticles. The effect is presumably due to interactions with plasmons on the metal surfaces and interactions between metals and adsorbed molecules.
Raman spectroscopy is a spectroscopic technique that produces results based on inelastic scattering of light interacting with molecules of interest. From an analytical standpoint the problem with Raman spectroscopy is its relatively poor sensitivity. However the sensitivity of the procedure can be enhanced through interactions with roughened metal surfaces or nanoparticles. The effect is presumably due to interactions with plasmons on the metal surfaces and interactions between metals and adsorbed molecules.