
Research Projects – Tiger Sharks

Click the image below to learn more about tiger shark effects on their prey and the Shark Bay community
Dolphins
Dugongs
Pied Cormorants
Indirect Effects of Tiger Sharks
Context Dependence of Predator Risk Effects
Effects of Tiger Sharks on Prey Diving Behavior
Sea Turtles
Sea Snakes
Prey Escape Tactics
Tiger Sharks

SBERP has been studying the tiger sharks (Galeocerdo cuvier) in Shark Bay since 1997. It is the perfect place for our study since it is one of the last places where a population of large sharks remains relatively free from human impacts. That means that we can use Shark Bay as a model to understand the role of tiger sharks in marine ecosystems and maybe learn how we can help to restore marine habitats where large shark populations have been dramatically reduced by human fishing.
On this page, you will learn how we study tiger sharks and some of the interesting things we have learned about their behavior. However, the most important things we have learned are how tiger sharks influence their prey and the role they play overall in the Shark Bay community. Click on a species below to learn about how tiger sharks influence them or the links to what we have learned about their overall role in the community.
Beyond the Body Count: Non-lethal Effects of Predators

When most people think of how predators influence their prey, they only think about how many prey are eaten. But predators can influence prey through more than direct predation because most animals have many behaviors that they use to stay safe. They may spend less time feeding and more time watching out for predators or they may spend their time in habitats where they are less likely to be eaten but they have less food available to them. These trade-offs between staying safe and getting food or finding mates mean that predators may actually regulate the populations of their prey more through the costly behavior they induce in their prey than the number of prey they kill. In Shark Bay, this certainly seems to be the case. Most of the prey we have studied are rarely killed by tiger sharks - but they play it really safe and have less opportunities to forage because of it. The pages below will show you how these species manage the risk from tiger sharks!
By studying so many individual species we have learned that not all species respond to tiger sharks in the same way. Instead, the responses depend on the context including the type of habitat and the escape behavior of each species.
Measuring Shark Abundance

research vessel
One of the most important components of our work is to monitor seasonal changes in the abundance of tiger sharks in the study area. By determining how their prey behave (what habitats they use, how they forage) when there are no sharks and then measuring how they change their behavior when tiger sharks are abundant in the area we can determine how prey respond to the risk of tiger shark predation and get a sense of how the ecosystem might change if tiger sharks were to disappear. This may not seem very likely in Shark Bay, but it is important to know because tiger sharks - like many other large shark species - are disappearing from the world's oceans quickly because of overfishing. The movie below shows how we catch, tag, and track tiger sharks in Shark Bay.
We document shark abundance using catch rates on drumlines. A drumline consists of a 4kg (9lb) boat anchor tied to a rope that is about 45m (150') long. At the end of the rope we attach three buoys, a drum full of air, and a 2m (6.5') chain that has a shark hook on the end. Because the bay is fairly shallow (10m or 33') the shark has plenty of line to swim around. We bait our lines with a small chunk of Australian salmon and check the lines every two hours to see what we have caught.

When there is a shark on the line we pick up the line and retrieve the anchor so we can drive where the shark wants to go. By letting the shark swim next to the boat we can work with it easily and minimize stress to the animal. All sharks are measured (total length - from the tip of the snout to the tip of the tail, fork length - from the tip of the snout to the fork of the tail, precaudal lenth - from the tip of the snout to the notch in front of the caudal (tail) fin) and then we put a numbered tag in the dorsal fin. We can determine whether a shark is a male or female by looking for the presence of

claspers which male elasmobranchs (sharks and rays) use in mating. Females don't have claspers. Before we remove the hook and release the shark we take a small sample from the shark's dorsal fin for genetic analyses and then roll the shark over and take several mililiters of blood from the caudal vein for our studies of shark predator-prey interactions (see below). Amazingly, when a tiger shark is rolled onto its back it goes into a state of "tonic immobility" that involves the shark not struggling or responding to handling which makes it a lot easier to collect a blood sample!
This video shows how SBERP researchers, catch, tag, sample, and track tiger sharks in Shark Bay (video by Patrick Greene Productions, 2009).
Measuring Shark Habitats and Movements

Originally, we had hoped to measure tiger shark habitat use by measuring catch rates in shallow and deep waters, but we quickly found out that large puffer fish and other smaller fish would eat our baits over seagrass beds before tiger sharks got to them! That meant we had to find other methods to determine which habitats sharks preferred.
Acoustic tracking and Animal-borne video and environmental data (AVED) systems were the perfect answer. In acoustic tracking, we attach an ultrasonic transmitter to the shark and then use a directional hydrophone to follow the pings the transmitter sends out once every second. We record the GPS location of the boat, direction and approximate distance to the shark, habitat, and water depth every five minutes. These data let us plot the approximate track of the shark. One problem is that we can't see exactly what type of area the shark is in or what it is doing. For that, we use AVEDs (including National
Geographic's Crittercam - pictured to the left). AVEDs combine a video camera and data sensors (depth and temperature) to show us exactly what the shark is doing and precisely where it is. The

AVEDs only stay on the animal for a few hours (usually around 6) and then release from the shark and float to the surface so we can get the data on the system. For our initial studies we tracked sharks for up to 18 hours and recorded up to 6 hours of video.
To determine where tiger sharks go when they are not inside our study area, we attached Wildlife Computers SPOT satellite transmitters to the dorsal fins of 4 sharks in 2004 and nine more in 2011. These transmitters relay the approximate GPS position of the sharks when the transmitter brakes the surface.
What We Have Found: Seasonal Changes in Tiger Shark Abundance

from the research vessel.
Tiger sharks are the most common large shark in Shark Bay - accounting for more than 95% of large shark catches. Since 1997, we have tagged and captured more than 600 sharks between 1.4-4.5m (4-15') long (average size about 2.9 m or 10'). Based on regurgitated prey items when sharks were next to the boat, some of the favorite prey items of tiger sharks in Shark Bay are sea snakes, sea turtles, and dugongs (for large sharks). Our studies using stable isotopes to unravel the trophic position of tiger sharks are still ongoing.
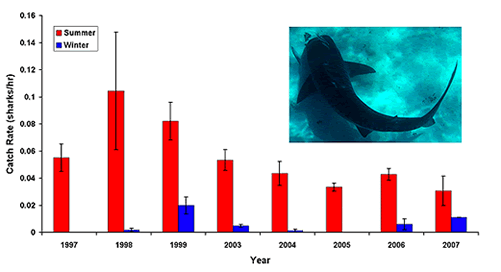
During the summers tiger shark abundance is incredibly high in our study area- on a good day we may catch seven sharks on just ten hooks - all before lunch. On average, shark numbers are much higher than other places where tiger sharks have been studied suggesting that Shark Bay provides an incredible opportunity for studying the ecological role of a large shark in a near-pristine setting. Sharks are not present in high abundances all year round, however. During the winter the number of sharks drops precipitously and they may be almost completely absent during some winters. Movements of sharks, however, are not linked exclusively to changes in water temperature. In general, there is more potential prey for tiger sharks in the summers and our catches of large sharks (over 3.5m, 11.5', long) are linked to the abundance of dugongs - a favorite prey item - in the bay.
Where do sharks go when they are not in the bay? Using satellite tags we found that there is no single answer. Of the four sharks we tagged in 2004, three moved along the coast of Western Australia, sometimes returning to Shark Bay. One shark, however, moved all the way across the Indian Ocean to the waters off of South Africa in just 99 days! Our sharks tagged in 2011 also showed very different movements. Some stayed near Shark Bay while others ranged widely along the Western Australian coast. Click here to see animations of the 2011 tracks!
What We Have Found: Tiger Shark Habitat Use

shallow seagrass bank -their
preferred habitat.
Our tracking and AVED studies have shown that tiger sharks in Shark Bay prefer shallow seagrass beds (usually <4m, 12', deep) over the surrounding deeper waters (6-10m, 20-33'). They like to swim along the edges of the banks the best, but they still prefer the middles of banks to the deeper waters. Why would a tiger shark want to spend time in shallow waters? Probably because shallow waters have a higher abundance of potential prey (especially sea snakes, dugongs, and turtles) than deeper waters. However, prey are not simply waiting around to get eaten - they have their own tactics to avoid becoming a meal and these tactics may actually help to shape the dynamics of the Shark Bay ecosystem. Also, we are

while on the dorsal fin a tiger shark
crusing over a shallow seagrass bed.
The shark's head is in the foreground
and a turtle is to the left of the frame.
investigating whether tiger shark preferences for shallow habitats - especially edges - might vary among regions with different mixes and configurations of deep and shallow waters. Click on the links above to learn more about the effects of tiger sharks on their prey and the Shark Bay community and the wider implications of these results.
Our videos from tiger sharks also gave us insights into their hunting behavior. Sharks do not appear to attack prey that notice them. AVEDs recorded a number of encounters between sharks and possible prey, but when the prey looked at the shark, the shark turned away not wasting the effort to attack something that might just get away. Also, we have seen that tiger sharks move up and down through the water column continuously. This may be a hunting tactic that lets them ambush surface prey and prey along the bottom.
Other Findings
Since 2005, we have been collecting tissue samples to conduct stable isotope analysis. More recently we started conducting fatty acid analysis. These biochemical techniques have shown us that, not surprisingly, tiger sharks are the top of the food web in Shark Bay. We also have found out that tiger sharks are true generalists both at the population level and within individuals. Tiger sharks do not appear to compete heavily with dolphins for fish prey.
Tiger Shark Studies Publications
- Heithaus, M. R., J. J. Vaudo, S. Kreicker, C. A. Layman, M. Krutzen, D. A. Burkholder, K. Gastrich, C. Bessey, R. Sarabia, K. Cameron, A. Wirsing, J. A. Thomson, and M. M. Dunphy-Daly. 2013. Apparent resource partitioning and trophic structure of large-bodied marine predators in a relatively pristine seagrass ecosystem. Marine Ecology Progress Series in press
- Heithaus, M. R., A. J. Wirsing, and L. M. Dill. 2012. The ecological importance of intact top predator populations: a synthesis of 15 years of research in a seagrass ecosystem. Marine and Freshwater Research 63: 1039-1050.
- Heithaus, M. R. and J. J. Vaudo. 2012. Predator-prey interactions. Pages 505-546 In: J. C. Carrier, M. R. Heithaus, and J. A. Musick (eds). The biology of sharks and their relatives, second edition. CRC Press.
- Wirsing, A. J. and W. J. Ripple. 2011. A comparison of shark and wolf research reveals similar behavioral responses by prey. Frontiers in Ecology and the Environment 9: 335-341.
- Wirsing, A. J and, M. R. Heithaus. 2012. Behavioral transition probabilities in dugongs change with habitat and predator presence: implications for sirenian conservation. Marine and Freshwater Research 63:1069-1076.
- Belicka, L. L., D. Burkholder, J. W. Fourqurean, M.R. Heithaus, S. A. Macko and R. Jaffé. 2012. Stable isotope and fatty acid biomarkers of seagrass, epiphytic, and algal organic matter to consumers in a nearly pristine seagrass ecosystem. Marine and Freshwater Research 63: 1085-1097.
- Wirsing, A. J., M. R. Heithaus, and L. M. Dill. 2011. Predator-induced modifications to diving behavior very with foraging mode. Oikos 120: 1005-1012.
- Matich, P., M. R. Heithaus, and C. A. Layman. 2011. Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. Journal of Animal Ecology 80: 294-305.
- Feretti, F. B. Worm, G. L. Britten, M. R. Heithaus, H. K. Lotze. 2010. Patterns and ecosystem consequences of shark declines in the ocean. Ecology Letters 13: 1055-1071.
- Matich, P., M. R. Heithaus, and C. A. Layman. 2010. Size-based inter-tissue comparisons of stable carbon and notrogen isotope signatures of bull and tiger sharks. Canadian Journal of Fisheries and Aquatic Sciences 67: 877-885.
- Wirsing, A. J., K. A. Cameron, and M. R. Heithaus. 2010. Spatial responses to predators vary with prey escape mode. Animal Behaviour 79: 531-537.
- Dunphy-Daly, M. M., M. R. Heithaus, A. J. Wirsing, J. S. F. Maradon, and D. A. Burkholder. 2010. Predation risk influences the diving behavior of a marine mesopredator. Open Ecology Journal 3: 8-15
- Wirsing, A. J. and M. R. Heithaus. 2009. Olive-headed sea snakes (Disteria major) shift seagrass microhabitats to avoid shark predation. Marine Ecology Progress Series 387:287-293.
- Heithaus, M. R., A. J. Wirsing, D. Burkholder, J. Thomson, and L. M. Dill. 2009. Towards a predictive framework for predator risk effects: the interaction of landscape features and prey escape tactics. Journal of Animal Ecology 78: 556-562.
- Heithaus, M. R., A. Frid, A. J. Wirsing, and B. Worm. 2008. Predicting ecological consequences of marine top predator declines. Trends in Ecology and Evolution 23: 202-210.
- Heithaus, M. R., A. J. Wirsing, J. Thomson, and D. Burkholder. 2008. A reveiew of lethal and non-lethal effects of predators on adult marine turtles. Journal of Experimental Marine Biology and Ecology 356: 43-51.
- Wirsing, A. J., M. R. Heithaus, A. Frid, and L. M. Dill. 2008. Seascapes of fear: methods for evaluation sublethal predator effects experienced and generated by marine ammals. Marine Mammal Science 24: 1-15.
- Kerfod, M. A., A. J. Wirsing, M. R. Heithaus, and L. M. Dill. 2008. Danger on the rise: habtiat use by bar-bellied sea snakes in Shark Bay, Western Australia. Marine Ecology Progress Series 358: 289-294.
- Heithaus, M. R. A. J. Wirsing, A. Frid, and L. M. Dill. 2007. Species interactions and marine conservation: lessons from an undisturbed ecosystem. Israel Journal of Ecology and Evolution 53: 355-370.
- Wirsing, A. J., M. R. Heithaus, and L. M. Dill. 2007. Can you dig it? Use of excavation, a risky foraging tactic, by dugongs is sensitive to predation danger. Animal Behavior 74: 1085-1091.
- Wirsing, A. J., M. R. Heithaus, and L. M. Dill. 2007. Fear factor: Do dugogns (Dugong dugon) trade food for safety from tiger sharks (Galeocerdo cuvier)? Oecologia 153: 1031-1040.
- Heithaus, M. R., A. Fird, A. J. Wirsing, L. M. Dill, J. Fourqurean, D. Burkholder, J. Thomson, and L. Bejder. 2007. State-dependent risk-taking by green sea turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. Journal of Animal Ecology 76: 837-844.
- Wirsing, A. J., M. R. Heithaus, and L. M. Dill. 2007. Can measures of prey availability improve our ability to predict the abundance of large marine predators? Oecologia 153:563-568.
- Wirsing, A. J., M. R. Heithaus, and L. M. Dill. 2007. Living on the edge: dugongs prefer foraging microhabitats that allow escape rather than avoidance of predators. Animal Behaviour 74: 93-101.
- Heithaus, M. R., A. J. Wirsing, and L. M. Dill. 2007. Long-term movements of tiger sharks satellite-tagged in Shark Bay, Western Australia. Marine Biology 151: 1455-1461.
- Wirsing, A. J., M. R. Heithaus, and L. M. Dill. 2006. Tiger shark (Galeocerdo cuvier) abundance and growth rates in a subtropical embayment: evidence from seven years of standardized fishing effort. Marine Biology 4: 961-968.
- Heithaus, M. R., I. M. Hamilton, A. J. Wirsing, and L. M. Dill. 2006. Validation of a randomization procedure to assess animal habitat preferences: microhabitat use of tiger sharks in a seagrass ecosystem. Journal of Animal Ecology 75: 666-676.
- Heithaus, M. R. and L. M. Dill. 2006. Does tiger shark predation risk influence foraging habitat use by bottlenose dolphins at multiple spatial scales? Oikos 114: 257-264.
- Heithaus, M. R., A. Frid, A. Wirsing, L. Bejder, and L. M. Dill. 2005. The biology of green and loggerhead turtles under risk from tiger sharks at a foraging ground. Marine Ecology Progress Series 288: 285-294.
- Heithaus, M. R. 2005. Habitat use and group size of pied cormorants (Phalacrocorax varius) in a sea grass ecosystem: possible effects of food abundance and predation risk. Marine Biology 147: 27-35.
- Heithaus, M. R., L. M. Dill, G. J. Marshall, and B. Buhleier. 2002. Habitat use and foraging behavior of tiger sharks (Galeocerdo cuvier) in a sea grass ecosystem. Marine Biology 140: 237-248.
- Heithaus, M. R., A. Frid, and L. M. Dill . 2002. Shark-inflicted injury frequencies, escape ability, and habitat use of green and loggerhead turtles. Marine Biology 140: 229-236.
- Heithaus, M. R. and L. M. Dill. 2002. Food availability and tiger shark predation risk influence bottlenose dolphin habitat use. Ecology 83: 480-491.
- Heithaus, M. R. 2001. Shark attacks on bottlenose dolphins (Tursiops aduncus) in Shark Bay, Western Australia: attack rate, bite scar frequencies, and attack seasonality. Marine Mammal Science 17: 526-539.
- Heithaus, M. R. 2001. The biology of tiger sharks (Galeocerdo cuvier) in Shark Bay, Western Australia: sex ratio, size distribution, diet, and seasonal changes in catch rates. Environmental Biology of Fishes 61: 25-36.
- Heithaus, M. R., G. J. Marshall, B. M. Buhleier, and L. M. Dill. 2001. Employing Crittercam to study habitat use and behavior of large sharks. Marine Ecology Progress Series 209: 307-310.
All photographs copyrighted; Images may be used for educational purposes. For use in other forms contact Mike Heithaus










